- Last updated:
- 08/29/2019
Accessing IU Health PHI/biospecimens for Research Purposes
- Guidance Contact:
IU Human Research Protection Program (HRPP)
irb@iu.edu
IU Human Research Protection Program (HRPP)
irb@iu.edu
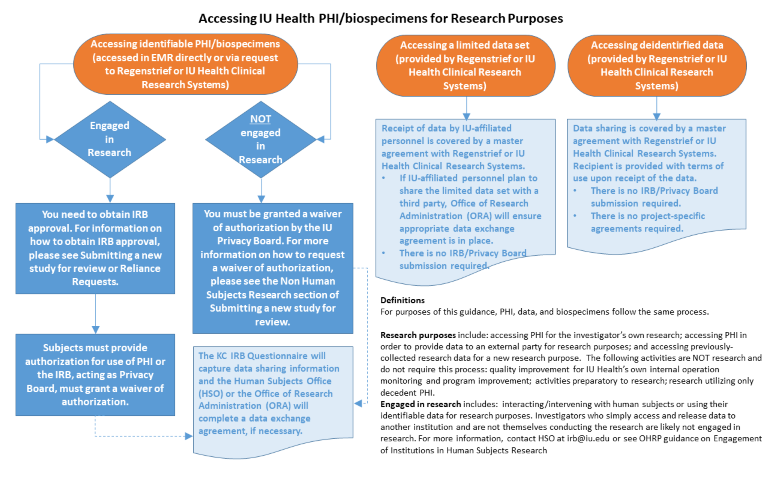
This guidance is intended to explain the specific requirements, e.g., approvals and/or agreements, to access identifiable protected health information (PHI) or biospecimens from IU Health for research purposes.
(accessed in EMR directly or via request to Regenstrief or IU Health Clinical Research Systems)
(provided by Regenstrief or IU Health Clinical Research Systems)
Receipt of data by IU-affiliated personnel is covered by a master agreement with Regenstrief or IU Health Clinical Research Systems.
(provided by Regenstrief or IU Health Clinical Research Systems)
Data sharing is covered by a master agreement with Regenstrief or IU Health Clinical Research Systems. Recipient is provided with terms of use upon receipt of the data.
For purposes of this guidance, PHI, data, and biospecimens follow the same process.
Research purposes include: accessing PHI for the investigator’s own research; accessing PHI in order to provide data to an external party for research purposes; and accessing previously-collected research data for a new research purpose.
The following activities are NOT research and do not require this process: quality improvement for IU Health’s own internal operation monitoring and program improvement; activities preparatory to research; research utilizing only decedent PHI.
Engaged in research includes: interacting/intervening with human subjects or using their identifiable data for research purposes. Investigators who simply access and release data to another institution and are not themselves conducting the research are likely not engaged in research. For more information, contact HSO at irb@iu.edu or see OHRP guidance on Engagement of Institutions in Human Subjects Research
Below is an infographic representing the information on this page.